a) Give a brief explanation of LT-FAB.
Low-temperature fast atom bombardment (LT-FAB) mass spectra are obtained during thawing of a frozen solution in the ion source of the mass spectrometer, thereby allowing to employ almost any solvent as matrix in LT-FAB-MS. Consequently, neither volatility nor unwanted chemical reactions with the matrix restrict the choice of a matrix. Instead, the solvent matrix may be tailored to the analyte’s requirements.
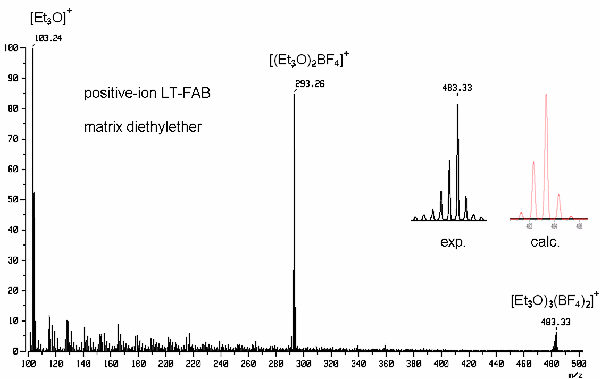
b) The positive-ion LT-FAB spectrum of a simple Meerwein salt (oxonium salt) with a boron-containing counterion is shown below. Assign formulas to those peaks with a mass label and identify the salt.
The peaks are 190 u distant and according to the general behavior of salts in FAB, this reflects the mass of the complete salt, because the peaks arise from clustering.
The peak at m/z 103 cannot correspond to a cluster ion, however, it must represent the salt’s cation instead. Thus, one can deduce that the counterion has a mass of 87 u, 11 u of which are covered by the boron atom. The remaining mass of 76 u can be explained by four fluorine atoms needed to complete a BF4– anion.
Subtracting 16 u for the oxygen from the oxonium ion’s mass, the remaining 87 u have to be filled up with alkyl substituents. The easiest explanation for the cation is Et3O+ (nonetheless, Me2BuO+ could also meet this spectrum). For chemical reasons, the alkyl group of the solvent ether must be identical to those of the oxonium ion.