a) What kind of ions do you expect to predominate with the following analyte/solvent systems in ESI? Complete the table.
polystyrene in toluene protein in water/acetonitrile 1:1 + 0.1 % TFA oligosaccharide in water/methanol 5:1 anionic transition metal complex in chloroform polycyclic aromatic hydrocarbons in hexane oligonucleotide in water/isopropanol + amines 50 mM peptides water/acetonitrile 1:3 + 0.5 % acetic acid does not spray [M+H]+, [M+alkali]+ plus analogous multiply charged ions [M+alkali]+ [A]– (plus cluster ions, e.g., [2A+C]– does not spray [M-H]–, [M-2H+alkali]– plus analogous multiply charged ions [M+H]+, [M+alkali]+ plus analogous multiply charged ions
b) A synthetic oligonucleotide 8mer has been analyzed by negative-ion ESI mass spectrometry on a linear quadrupole mass spectrometer. The isotopic peaks are not resolved. Determine the molecular weight of the analyte. Follow your intuition.
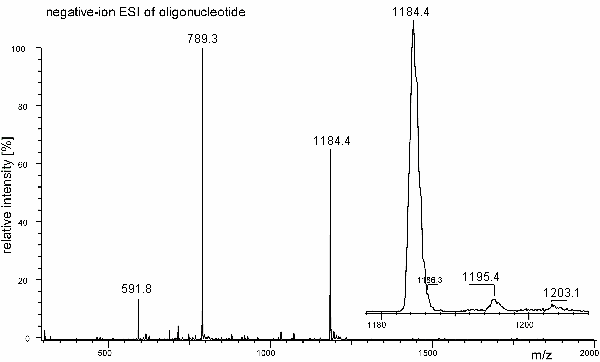
The key step for mass assignment is to recognize that the signal at m/z 1184 refers to the doubly charged negative ion. The first argument comes from the mass of an average nucleoside which is about 300 u, thereby yielding a rough estimate of 2400 u for the 8mer. Second, its accompanying peaks are at plus 11 u and plus 18.7 u which upon multiplication by a factor of 2 corresponds to plus 22 u and plus 38 u as known to result from exchange of hydrogen for sodium or potassium.
Thus, we have a set of three m/z values for determination of Mr:
Mr = 2 * 1184.4 u + 2 u = 2370.8 u
Mr = 3 * 789.3 u + 3 u = 2370.9 u
Mr = 4 * 591.8 u + 4 u = 2371.2 u
which gives us
Mr = 2371.0 u on the average.
c) Use the m/z 1184.4 and m/z 789.3 pair of peak to feed the charge deconvolution algorithm. Calculate the molecular weight again.
The first peak is at m/z 1184.4, the second at m/z 789.3. Now, the charge state n1 of the first ion is obtained according to Eq. 12.9 (after adaptation to negative ions) from:
n1 = (789.3 +1) / (1184.4 – 789.3) = 790.4 / 395.1 = 2.
Next, Mr is calculated from Eq. 12.10 (after adaptation to negative ions):
Mr = 2 * (1184.4 u +2) = 2370.8 u
The value of 2370.8 u nicely corresponds to the result Mr = 2371.0 u in part c).