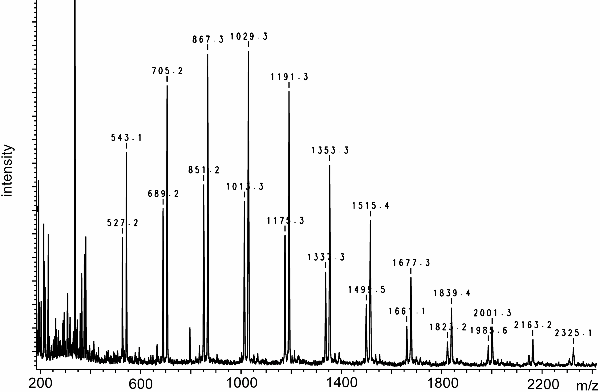
The following MALDI spectrum has been obtained from a water-soluble biopolymer in onions. It has been acquired in the positive-ion mode and 2,5-DHB was used as the matrix. The isotopic pattern is not resolved.
a) What kind of biopolymer do you expect? Use the mass difference between peaks to identify the compound class.
There are two series of peaks, one starting at m/z 527.2 the other at 543.1. The peaks of each series are separated by 162 u which is typical for oligosaccharides and corresponds to the C6H10O5 monomer units.
b) Identify what kind of ionic species are occurring here.
Oligossaccharides preferably form [M+alkali]+ ions. Therefore, the difference of 16 u between peaks of either series is most probably due to the mass difference between [M+Na]+ and [M+K]+ ions (cf. Downloads area).
c) Now you can also try to identify the endgroups.
Stepwise subtraction of 162 u, e.g., three times in case of m/z 543.1, leads to a rest of 57.1 u, 39 u of which are due to the K+ ion. The remaining 18.1 u are in good accordance with the formula weight of H2O, i.e., we simply have H on one and OH on the other end of the carbohydrate chain.
The spectrum shows the fructans present in onions (cf. Anal. Biochem. 1997, 246, 195-204).