a) What kind of ions do you expect to predominate with the following analyte/solvent systems in ESI? Complete the table.
polystyrene in toluene protein in water/acetonitrile 1:1 + 0.1 % TFA oligosaccharide in water/methanol 5:1 anionic transition metal complex in chloroform polycyclic aromatic hydrocarbons in hexane oligonucleotide in water/isopropanol + amines 50 mM peptides water/acetonitrile 1:3 + 0.5 % acetic acid ….. ….. ….. ….. ….. ….. …..
b) A synthetic oligonucleotide 8mer has been analyzed by negative-ion ESI mass spectrometry on a linear quadrupole mass spectrometer. The isotopic peaks are not resolved. Determine the molecular weight of the analyte. Follow your intuition.
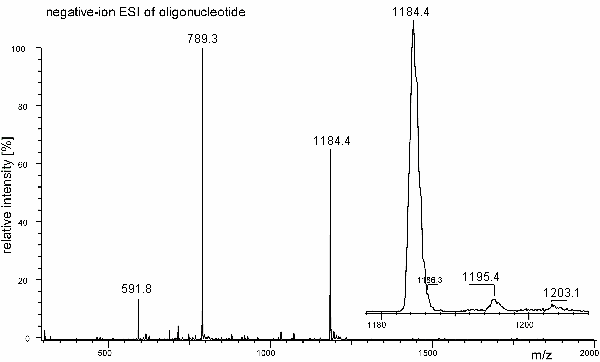
c) Use the m/z 1184.4 and m/z 789.3 pair of peak to feed the charge deconvolution algorithm. Calculate the molecular weight again.
Answered all questions? Want to see the solutions page?