a) What polarity and what kind of analyte ions do you expect to predominate with the following analytes in MALDI? Adjust the second column correctly.
Polystyrene Protein Oligosaccharide Fullerene derivative Polycyclic aromatic hydrocarbon Oligonucleotide Peptide [M+Ag]+ [M+H]+, [M+alkali]+ [M+alkali]+ M–. M+. [M-H]–, [M-2H+alkali]– [M+H]+, [M+alkali]+
b) The following MALDI spectrum has been obtained from a sample which was assumed to be C120OS, a dimeric fullerene derivative where the bucky balls are linked via O and S.
It has been acquired in the negative-ion mode and nitroanthracene was used as the matrix. The isotopic pattern is not resolved.
Assign composition and charge to the signals.
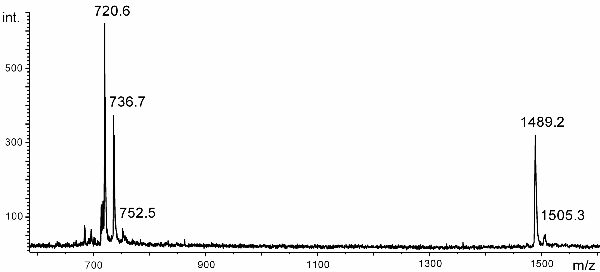
The peaks (from low to high m/z) correspond to fragment ions C60–, C60O–, C60S– or C60O2– (presumably impurities from oxydation), C120OS-. and C120O2S-. (impurity from oxydation, cf. Chem. Commun., 1999, 465-466).
c) Calculate the theoretical m/z values of the ions in the above spectrum.
As the isotopic pattern is not resolved, atomic weights have to be used for calculation rather than isotopic masses. Thus, we obtain 720.66 for C60–, 736.66 for C60O–, 752.72 for C60S– or 752.66 C60O2–, 1489.38 for C120OS-. and 1505.38 for C120O2S-..