a) What sort of ions does FD create from unpolar analytes?
Nonpolar analytes preferably form M+. ions in FD-MS.
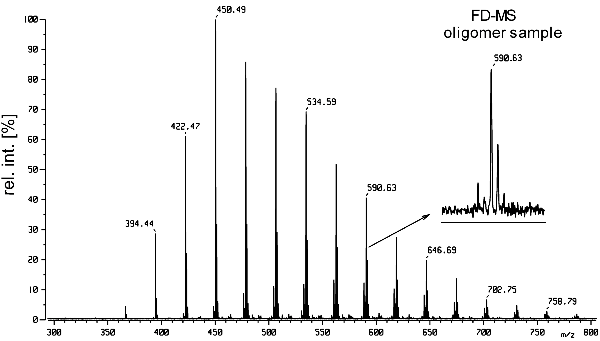
b) The FD mass spectrum below has been obtained from an oligomer moderately soluble in xylene. Identify the oligomer and its end groups.
The prominent peaks may be assumed to represent molecular ions of the corresponding oligomer molecules, whereas the formation of quasimolecular ions is much less probable with an analyte of low polarity. The average spaces between the signals are about 28.02 u which corresponds to -CH2CH2– units. Thus, the oligomer appears to be of polyethylene type.
Finally, one may assume hydrogen as the endgroups in PE. This can be checked by subtracting 2 (mass of 2*H) from one or some of the lower m/z values and subsequently dividing the residue by 28.02, e.g., (394.44-2)/28.02 = 14.005 and (422.47-2)/28.02 = 15.006.
Indeed, this is the FD mass spectrum of PE500, H-(CH2CH2)n-H, and the first prominent peak at m/z 394.44 corresponds to the n = 14 oligomer.